Products
Our biomaterials solve problems that have long challenged medicine
Our arsenal of innovative biomaterials provides local therapy for important medical conditions across therapeutic areas. Although our products differ in application and material, all require a thoughtful design of chemistries, consideration of the biological environment, and precise engineering of enabling delivery devices.
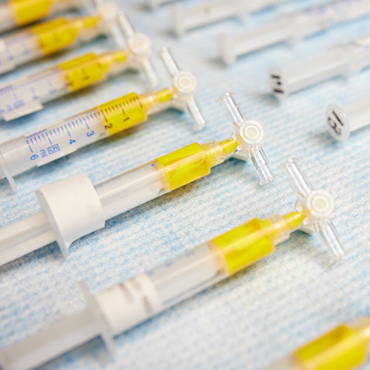
NeoCast™
An injectable, shear-responsive material that enables deep neurovascular occlusion
Injected through a microcatheter, NeoCast is a shear-responsive biomaterial that is designed to flow deeply to the microvasculature, providing robust occlusion in interventions including middle meningeal artery embolization (MMAe) for chronic subdural hematoma (cSDH), pre-surgical embolization of brain tumors and other neurovascular conditions.